News
New Building Material Developed at the University of Tokyo Updated in June 2021
The University of Tokyo announced that it has developed a building material by directly bonding sand with a catalyst, rather than traditional adhesive components like cement or resin. Materials primarily containing silicon dioxide (SiO2), such as sand or gravel, can be used. If put into practical use, it will solve problems arising from shortages of raw materials used to make concrete. It will also suppress energy consumption during manufacturing because it can be processed at low temperature.

Left: Silica sand Right: Desert sand
(provided by Sakai Yuya Laboratory, University of Tokyo)
Concrete, a ubiquitous building material, is generally produced by adding cement and water to sand and gravel. The cement and water act to bond the sand and gravel, giving concrete its strength. However, the shortage of appropriately sized sand and gravel, plus high-quality limestone─the main raw material for cement─has become a global issue. There are concerns about the long-term impact on construction and civil engineering work. In addition, a large amount of carbon dioxide (CO2) is generated in the production of cement. There is a demand for building materials that can be manufactured with less energy by using undepleted raw materials.
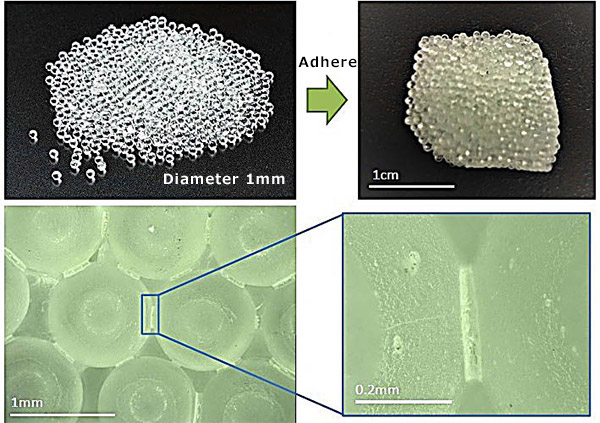
Under these circumstances, Associate Professor Sakai Yuya (Concrete Engineering) of the Institute of Industrial Science, University of Tokyo, is researching the development of next-generation building materials. He made a liquid combining alcohol and potassium hydroxide (KOH) and heated it to 240 degrees Celsius in a closed container. By cooling it down to room temperature, his team succeeded in making an artificial hard stone. It does not require a binder like cement. Adding heat to the mixture breaks the chemical bonds in the sand, and cooling it can make the sand bond together. The team was able to create hard stones from materials containing SiO2 as the main component: silica sand, glass, desert sand, and materials similar to lunar sand.
Temperatures of 1450 degrees Celsius are needed to make cement. Even the process of melting and bonding sand itself requires a heat of 1000 degrees Celsius or more. This can consume large amounts of energy. The new technique, on the other hand, succeeded in significantly lowering the required temperature. What’s more, the left-over alcohol and catalyst can be reused repeatedly.
While the durability of the new material surpasses concrete, its strength is about one half of concrete. However, in the future the strength of the material can be improved through innovations in the type and amount of catalyst, the size of sand grains, and the heating time and temperature. Research will continue to aim to further lower the heating temperature.
SiO2 is the main component of many types of sand and gravel, and is also contained in waste glass. Through this technology, spherically shaped small sand and gravel that previously could not be used to make concrete can now be used to avoid resource depletion. SiO2 is also the main component of sand on the Moon and Mars, and in the future we may be able to source building materials locally and use them to construct base stations on the surface of celestial bodies.
Associate Professor Sakai said, "It helps to solve the growing global concern about the shortage of raw materials for building materials, and can reduce energy consumption and CO2 emissions. It is a technology that can play a major role, particularly in space development." The results were announced by the University of Tokyo on April 14, 2021 and will be published in the May 1 issue of the institute’s research bulletin,