News
From Sea Water to Drinking Water: Resolving World's Water Shortage Updated in April 2020
Our Earth is often called the Planet of Water. About 70 percent of the surface is covered in water, most of which in the form of seawater and glaciers, with only 0.01 percent of freshwater available for living and industrial use. Under JST's Center of Innovation (COI) program, Shinshu University Global Aqua Innovation Center is developing a water treatment membrane that can remove impurities contained in water using nanocarbon fibers and material separation technology. The project is run through an industry-academia-government collaboration, and aims to produce freshwater from seawater, to purify contaminated water, and to create a future where everyone everywhere can access an abundance of clean water.
Nanocarbon─The Key to Solving Water Shortages
Water is indispensable for human life. Demand for water increases as populations grow, as the climate changes, and as economies develop. Effective use of limited water resources is an urgent issue. At present, more than 1.1 billion people, especially in Africa and Asia, are not able to secure safe drinking water. There is a particular shortage of agricultural water—approximately 900 million people are malnourished due to insufficient access to food. Also needed are more advanced solutions to dealing with water pollution from industrial wastewater and resource production sites.
“The goal is to develop affordable technology that produces clean fresh water from a variety of sources, and to build a society with a sound water cycle to support safe and healthy living,” said Project Leader and Technology Advisor Dr. Tsuzuki Koichi of Shinshu University's COI (Center of Innovation) hub. “Much more water available if we can efficiently remove impurities from seawater and groundwater. Unsanitary water kills; so much so that it is estimated that average life expectancy will grow by two years if we can just secure more clean water for the world.”
One key technology that the JST-funded research hub at Shinshu University is developing is a water treatment membrane using nanocarbon. Nanocarbon is a very tough, state-of-the-art carbon material with excellent electrical, thermal and chemical properties. The research team is led by Shinshu University Specially Appointed Professor Dr. Endo Morinobu. He is one of the first to pioneer mass production technology for carbon nanotubes (CNTs), a type of nanocarbon.
“My intuition really pushed me to make major technological innovations in nanocarbon film research,” he said.
At the Shinshu University site, companies handling nanocarbon materials, water treatment membranes, desalination plants, and research institutions specializing in computer chemistry are all participating in this project. By combining the world's best technologies, development is underway for innovative, functional water treatment membranes and systems in which they can be used. The ultimate goal is “Aqua Innovation” (Fig. 1).

At the Shinshu University International Science and Innovation Center, which is the core facility at the COI hub at Shinshu University, an overview of the hub and research results are introduced on videos and panel displays. In the foreground is a diorama depicting the futuristic “Aqua Innovation”: Seawater desalination, associated water treatment, irrigation treatment, and water treatment regeneration facilities can be installed on arid desert land, and commercial areas with high-rise buildings, resorts, residential areas, and agricultural areas can be subsequently developed to make up a sustainable city.
A Strong Film to withstand Clogging from Impurities
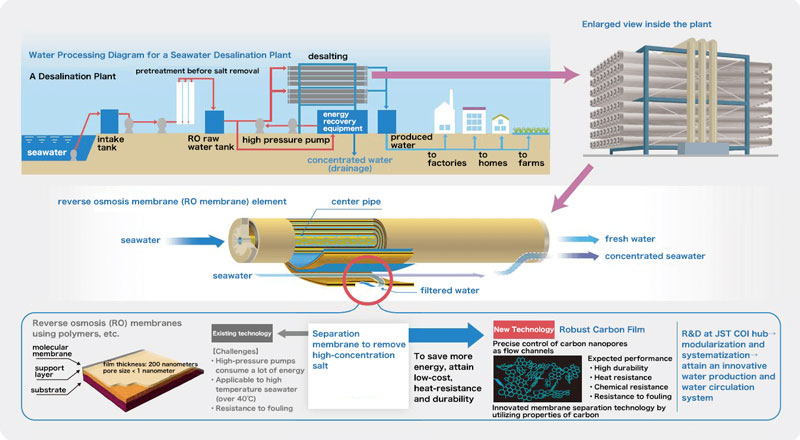
Seawater accounts for over 97% of the Earth's water. One desalination technology, the RO (reverse osmosis) membrane method, utilizes the principle of osmotic pressure. Membranes with countless fine pores only allow water molecules to pass through, allowing impurities like salt to be filtered from seawater (Fig. 2).
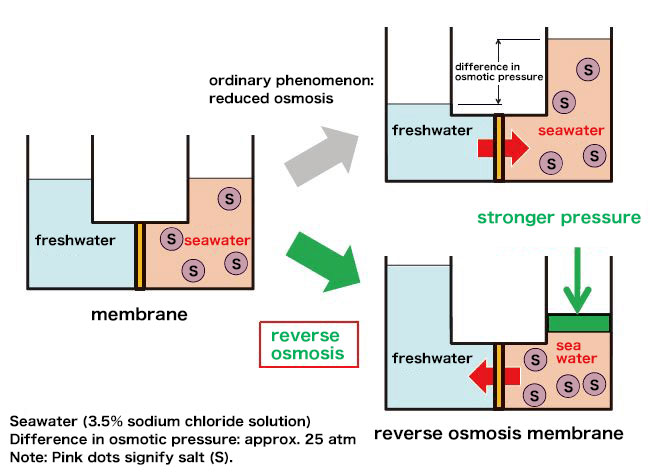
A semipermeable membrane with pores about 0.5 nanometers in diameter (a nanometer is one billionth of a meter) allows water molecules to pass through while blocking impurities. When freshwater and seawater are respectively supplied to the left and right sides of the membrane, water molecules contained in the freshwater-side (blue) can flow into the seawater-side (peach) to reach an equilibrium concentration. At this point, osmotic pressure corresponding to the concentration level occurs in the membrane. By applying higher pressure to the seawater-side, the water molecules in the seawater pass through the membrane to the freshwater side. This phenomenon is called “reverse osmosis.”
The RO membrane method has become the mainstream technology in seawater desalination. Seawater desalination plants using RO membranes are popular especially in Middle Eastern countries, many of which have few natural freshwater resources. But there are drawbacks—one is called fouling, which happens when membranes become clogged over time as non-salt impurities like seawater minerals and microorganisms accumulate. This causes a decline in the system's ability to produce fresh water. Regular cleaning is therefore necessary, but chlorine agents can damage the film. Another drawback is that a large amount of electricity is needed to produce the pressures required to filter seawater; thus, dissemination of the technology in developing countries and regions has been slow. Expectations are high for a durable or robust membrane resistant to dirt, and a treatment system that can desalinate at low cost.
Experiments and Computer Chemistry Help Create Guidelines for Material Design
Carbon nanotubes (CNT) are expected to play a key role in suppressing fouling. Polymer film material called polyamide (PA) is typically used in creating RO film. Mixing in CNT changes the pore structure and the electronic state of PA, which prevents the intrusion of salts and allows more water to pass through.
However, previous experiments have only been able to mix in CNT at a rate of 0.1%. It was extremely difficult to disperse the CNTs and mix them evenly throughout the PA material.
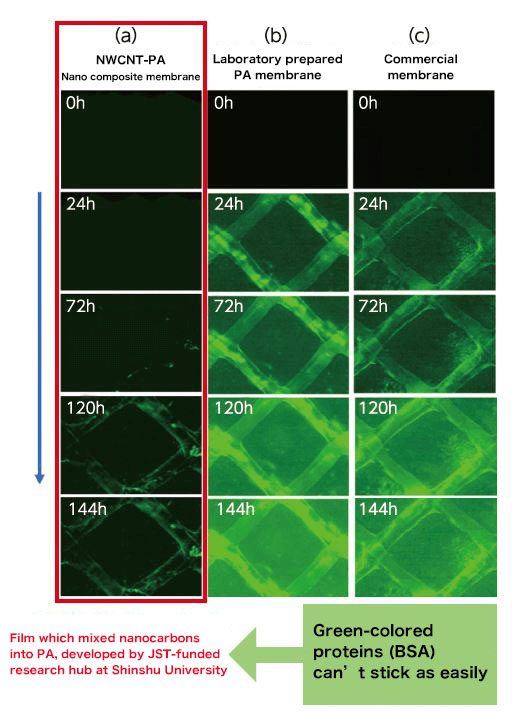
“By only mixing in a small amount of CNTs, we cannot benefit from CNTs' excellent properties,” says Dr. Endo. “I thought that if we mixed in more, we could make a more durable membrane.” By combining technology that detangles CNT fibers with conventional thin-film-forming tech, the team succeeded in developing and patenting a unique nanocomposite film-forming technology. In 2015, the team succeeded in creating a nanocarbon composite RO membrane which can evenly disperse CNTs in PA at a rate over 100 times higher than previous products. As predicted by Dr. Endo, not only was it possible to obtain high resistance to fouling and high water-permeation, but it was also found to be resistant to chlorine (Fig. 3). Since the product life is now prolonged, maintenance costs can be reduced. Since its launch in 2013, Shinshu University has published numerous papers on such high-performance composite membranes.
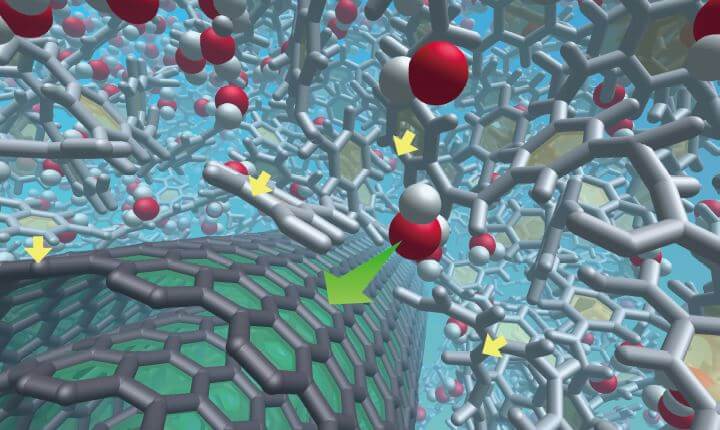
Water molecule movement in a nanocarbon composite RO membrane. When CNTs are mixed in, PA molecules are arranged along the CNTs (yellow arrows), and water molecules move along the CNTs (green arrows), improving water permeability. Salt removal rate is improved because fewer impurities enter the film thanks to the charging and transferring of electrons from the PA to the CNT.

Production line for nanocarbon composite RO membranes, located at Shinshu University International Science Innovation Center.
In order to obtain a higher-performance film, Dr. Endo surmised that it would be necessary to know how water molecules and ions interact with the CNT film at the atomic level aside from the foul-resisting and chlorine-resisting mechanisms. Thus, a supercomputer was used to simulate the precise behavior of water molecules as they interacted with the composite membrane (Fig. 4). It was found that water molecules formed an ultra-thin layer called “interfacial water” on the film's surface, which acted as a cover to prevent an assortment of impurities from sticking to the membrane. In addition, the decreased pore size of the film prevented penetration from further impurities. The results were consistent with experiments using real materials, including the PA molecular arrangement around the CNTs, and the effectiveness that smaller pores had on preventing the intrusion of impurities.
“By introducing computer chemistry for the first time, it has become possible to quickly determine guidelines for material design, in addition to doing the complex analysis of nanocarbon films, both of which were difficult in the past. Now we are pursuing R&D based on both experimental and computational chemistry to set guidelines for film design,” Dr. Endo says. A more precise material design has become possible. In the future, a high-performance film will be stable in several different types of water. The goal is to improve the membrane's functionality so that it can be used in next-generation water treatment membranes with wider scopes for application.
Pilot Test for Practical Use
“Nanocarbon composite RO membranes are so robust that there is plenty of room to enter the market,” says Project Leader Dr. Tsuzuki. For practical use, the team is working on prototypes that are similar to products at their own manufacturing facilities (Fig. 5).
In April 2019, a pilot test for a seawater desalination system using the nanocarbon composite RO membrane was launched at Water Plaza Kitakyushu in Fukuoka Prefecture (Figure 6). It aims to solve systemic problems by modularizing the composite membrane and incorporating it in a pilot plant. “Since the state of the sea changes greatly depending on the season, we plan to conduct a pilot test over the course of a year. By using real seawater, we will evaluate the performance of the composite membrane and discuss potential obstacles,” Dr. Tsuzuki explained.
Dr. Endo also has high hopes for this pilot experiment. “Unless you are fully committed it will be impossible to collaborate with the private sector to put this technology to practical use. We must come up with great results in terms of both performance and cost to comply with strict corporate standards.” Estimates show that using composite membranes can cut seawater desalination costs by 37% and water processing costs by 25% compared to conventional desalination systems.
Contribution to the local community is also one of the university's missions. Dr. Tsuzuki proposes to “dig around for new market needs by installing a platform at our hub and making ourselves available to local companies who want to take part in this project,” proposes Dr. Tsuzuki. Recently, this technology is under consideration for use in the food industry to improve concentration processes.
Utilizing a Variety of Water Sources
A New Industry from Japan
In the future, experts in the field will develop a water treatment system using nanocarbon composite RO membranes not only for seawater, but also to separate out oils derived from mining fossil fuels or natural gas, or materials that are commonly found with water, including organic matter, brine/saltwater from lakes, marshes or groundwater, domestic/industrial wastewater or sewage.
One example is an adsorbent that uses molten salt nanomaterials to remove harmful heavy metals from groundwater. Since 2018, a water quality survey has been conducted in Tanzania (Fig. 7). “In Tanzania, geological formations have caused fluorine to enter groundwater in some areas, causing health problems to those who drank it,” says Dr. Tsuzuki. Fluoride binds to calcium and thus affects bone growth. Similar problems have occurred in various countries and regions like India and Bangladesh. “We've begun a joint project with a university in Tanzania to remove fluorine in groundwater and create safe drinking water, using technology from the JST-funded research hub at Shinshu University. We are also working on a joint project to train scientists and technicians to ensure water quality.”
Dr. Endo speaks passionately about his future dreams. “We are trying to commercialize this water treatment system quickly and to establish it as a new industry from Japan.” Nagano Prefecture, the home of Shinshu University, is the source of eight rivers that provide top quality drinking water. "Because we were born and raised in an area blessed with such water resources and know what really good water tastes like, we want to deliver clean and safe water to people all over the world,” he says. His strong determination is what drives the R&D of this project.

A pilot plant for seawater desalination. A desalination device equipped with a nanocarbon composite RO membrane module, a chemical unit for evaluating membrane durability, a water tank, and more are installed in a 12-meter long container. High pressure is applied to the seawater to remove dirt and that water is passed through a membrane module to remove salt.

In Tanzania, local students conducted a water quality survey of groundwater/well-water and conducted a water purification experiment.