News
National Cancer Center Confirms Effectiveness of the miRNAs Test: Test can Spot 96% of Early Esophagus Cancer with few Drops of Blood Updated in September 2019
National Cancer Center Japan (NCC) recently announced that by looking closely at minute biological particles in the blood, called Micro RNAs or miRNAs, they were able to spot 96% of patients with esophageal cancer. NCC has already devised an innovative screening method to diagnose 13 types of cancer using MiRNAs. NCC is aiming for clinical use and is researching further into solutions for cancers located in different parts of the body. This time, the results indicated that practical application of this screening method was successful for cancer in the esophagus. If put into clinical use, this screening method will prove more effective than the conventional method using tumor markers.
The research group consists of members at various clinical and research departments of NCC and its central hospital. The team focused on MiRNAs secreted into the bloodstream from cancer cells. For each type of cancer, the group found that distinct MiRNAs would get secreted into the bloodstream. By monitoring the different types of MiRNAs and the change in the secreted amount, researchers could tell whether a cancer is formed or not. Only a few drops of blood are necessary to conduct this test. The following 13 types of cancer─the most common in Japan─are under further research for future clinical use: gastric cancer, esophageal cancer, lung cancer, liver cancer, biliary tract cancer, pancreatic cancer, colorectal cancer, ovarian cancer, prostate cancer, bladder cancer, breast cancer, sarcoma and glioma.
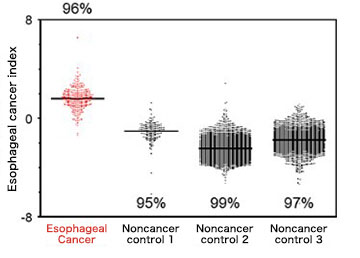
The research group focused on esophageal cancer this time. A total of 5,531 blood samples from 566 esophageal cancer patients and 4,965 noncancer samples were used to examine the characteristic of MiRNAs in esophageal cancer. As a result, the team found 6 types of MiRNAs that can screen whether the patient has cancer or not. By a new test method to monitor the amount of secreted Micro RNSs, the team confirmed that they were able to specify 96% of patients with esophageal cancer. Throughout early Stage 1 to progressed Stage 4, it was possible to diagnose the patient at a rate of 95%-100%.
By using the miRNAs method, NCC said it had already succeeded in spotting 99 % percent of patients with ovarian cancer. In collaboration with test-kit manufacturers, NCC is continuing this research to enable clinical use of the miRNAs method for other types of cancers.
MiRNAs can be found in bodily fluids like blood, saliva or urine. It consists of about 22 smaller RNAs called nucleotides. Humans are said to have about 2,500 kinds of miRNAs. Cells in our body pack these miRNAs into “small bags” and send them out into our bloodstream. Cancer cells can also release peculiar “evil-causing” miRNAs which can affect the progression of cancer.
The conventional tumor marker detects proteins released by human cells when they turn cancerous. Therefore, it was not possible to specify cancerous cells until they had progressed to a certain extent. Spotting the cancer much earlier had always been a challenge.
Cancer of the esophagus is more common among men and its progression is relatively fast. Therefore, it is crucial to find its signs as early as possible. Alcohol and smoking can greatly influence esophageal cancer. The latest survival data for 10 years is 30%. While survival rate at Stage 1 is 65%, only 7% may survive at Stage 4. Early detection and treatment are key.
“Patients can overcome esophageal cancer as long as it is detected early. This time, by using minimal amounts of blood, our research showed great accomplishment in the early detection of patients without symptoms. With more efforts, we hope that the miRNAs method will become a prominent diagnostic marker useful in the clinical field,” commented Dr. Ken Kato, head of gastroenterology at NCC’s central hospital.
JAMA Network Open:
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2734072
National Cancer Center Japan
https://www.ncc.go.jp/en/about/NCC_Introductory_Overview.pdf