News
Osaka University Develops New Immunotherapy for Blood Cancer on Mouse Updated in January 2018
On November 7, Osaka University group announced that through mouse experiment, they succeeded in developing a new immunotherapy which will only target typical blood cancer cells called Multiple Myeloma (MM). The group aims to put it into practical use through clinical research. On November 6, the paper was published on the electronic version of Nature Medicine.
Most MM patients are over 40 years or older. Even under chemotherapy, this type of cancer is hard to cure. According to the research group, there are about 18,000 patients in Japan.* According to National Cancer Center Japan, the 5-year survival rate is around 30 percent which is quite low. New treatments are sought after to counter growing number of patients in Japan’s aging society. *approx. 114,000 patients worldwide (source: International Agency for Research on Cancer IARC-GLOBOCAN 2012)
Research team led by Associate Professor Naoki Hosen at Osaka University Graduate School of Medicine found that “integrin β7,” a type of protein had or increased abnormally* and had turned into an activated structure on the surface of MM cells. Furthermore, they found that an antibody called MMG49 would specifically bind to integrin β7. Integrin β7 can be found on surfaces of normal blood cells, too. However, unlike the surface of MM cells, it did not display any activated structure. *Showed “high expression”
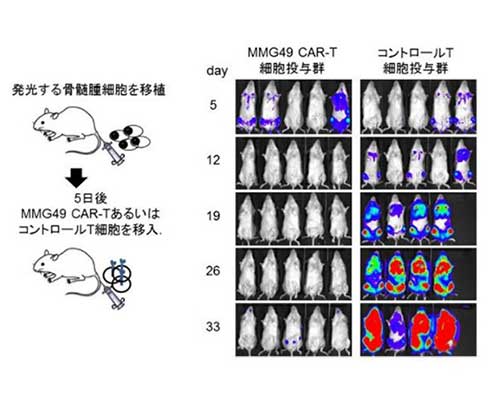
Based on such findings, the research group decided to genetically manipulate cancer-fighting T cells to establish CAR-T Cell Therapy which has more enhanced cancer-fighting power. CAR-T Cell Therapy is being applied to the treatment of Lymphocytic Leukemia (B lymphoid malignant tumor) and has proven effective. The group went on to combine CAR-T Cells and MMG49 to produce an artificial antibody cell named MMG49/CAR-T Cells. The team had transplanted MM cells to 16 mice and administered MMG-CAR T Cells to these mice after five days.
As a result, MM cells had dramatically decreased after one month of administering MMG49/CAR-T Cells, whereas MM cells had increased with passing of time in the control group of mice that did not get the antibody and some had started to die. Under further observation, 12 out of 16 mice had survived after two months in the group administered with MMG49/CAR-T Cells while 16 mice in the control group without antibody had all died within 40 days. With these results in hand, the group named this medical procedure “MMG49/CAR-T Cell Treatment.”
“Through doctor-initiated clinical trials, we will clarify whether the new immunotherapy develop this time will truly benefit patients,” commented Associate Professor Hosen.
https://resou.osaka-u.ac.jp/en/research/2017/20171107_3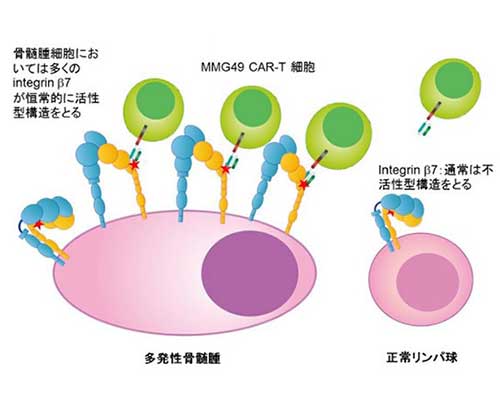
Photos courtesy of Japan Science and Technology Agency
Photos courtesy of Japan Science and Technology Agency







